the end of this year, "NASA opening press" seems destined to become an idiom, at 3:00 in the early morning of December 3, 2010 Beijing time, NASA at its Washington headquarters once again announced a "shocking world" (its real original is exceptional, extraordinary). News: we have found exactly what is important to space biology on the earth, in the Mono Lake (Mono Lake) in California, America: a exceptional bacterium, which we can call "arsenic based life". The lake water of
is very high in salinity and alkalinity, and is also one of the highest levels of arsenic in natural waters in the world. This extreme habitat has created many living organisms that tolerate extreme environments. And the NASA - sponsored scientist Felisa Wolfe-Simon, found in the lake bottom and the GFAJ-1 strain developed in the laboratory, belongs to the Proteobacteria, a step further than the simple tolerance environment, using the substance it should bear as the basis for its own life: it is used for most biological toxic arsenic. As a substitute for phosphorus, it becomes the basic element of cells.

Felisa successfully uses arsenic - cultured GFAJ-1 strains, and radioisotope markers confirm that they make up their own (NASA)
so GFAJ-1 is indeed an alternative to earth's life, but what this has to do with NASA's space biology ? The exceptional of GFAJ-1 is that it first confirms the idea that the essential elements of life can be replaced, so that the extraterrestrial environment, which was described as "unsuitable for life", may need to be reexamined, and they may actually be a fertile ground for the local life.
CHONPS, the basic element of life overturned, does it look familiar? This is not a word. It is the six basic elements of life that we recite in a secondary school biology textbook: carbon, hydrogen, oxygen, nitrogen, phosphorus and sulfur, which together make up the substances needed by the organism: nucleic acids, proteins, carbohydrates, lipids. In fact, for these six, we may have thought of "Peter to replace": we have seen the word "silicon based life" many times in science fiction: the silicon under the carbon on the periodic table, instead of carbon, becomes the cornerstone of life. However, the similar substitution, first confirmed by science, occurred in the fifth place phosphorus instead of the carbon, we guessed the beginning, but did not guess the end.
a review of the role of phosphorus in life:
DNA, which is the basis of genetic material, is called deoxyribonucleic acid, and its basic unit is a molecule of phosphoric acid linked to a molecule of five carbon atoms, plus a base. Well, what I'm talking about, the chemistry will not be back to the teacher. Please look at the following figure: a broken DNA double helix. The important thing is that two long chains of DNA are connected by the phosphoric acid molecules in the red circle. A section of
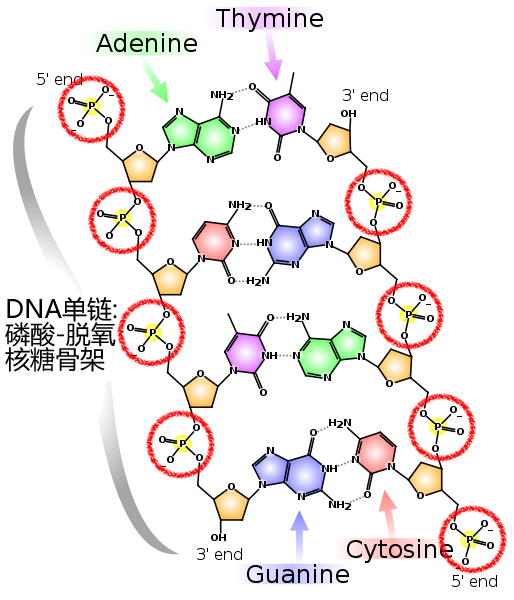
DNA, the double chains are bound together through the middle base pairs, and the red ring marks the important position of phosphoric acid as a long chain skeleton
to continue to recall the middle school biology, to transcribe the genetic code and translate it into the RNA of the protein, whether it is a letter. The transcriptional code is the ribosome of a protein factory. Its single stranded structure is similar to that of DNA, and is also linked by phosphoric acid as a basic framework.
perhaps quite a few people still remember the name of ATP, the universal energy currency in biology. Its full name is adenosine triphosphate. In addition, it may be remembered that the membrane consists of the phospholipid bimolecular layer, phosphorylation and phosphorylation, as well as the kinases of various phosphoric acid.
so, if the new research tells you that all these phosphorus words can be changed into arsenic, the red circle and the P in ATP can be written as As, and scientists are going to be full of cattle, you know.
why is arsenic substituted for phosphorus?
why is it not silicon instead of carbon? The simple and direct reason is that the GFAJ-1 strain was found in a lake with a lot of arsenic and phosphorus.
but we can also consider this problem in a large environment like the earth: carbon dioxide in the atmosphere, even in the form of methane at the beginning of the birth of life, and the carbonates in limestone are everywhere, and silicon exists widely in rocks in the form of two oxygen silicon. But their chemical properties are very inactive and difficult to be utilized by most organisms. What about
and the situation of phosphorus? Phosphorus almost does not exist in the atmosphere and water, and the natural phosphorus single substance does not exist (because everyone knows "spontaneous combustion"), and the phosphorus on the earth is mainly stored in phosphate ores. The chemical properties of phosphate are very stable, which is why our bones have calcium phosphate, because calcium phosphate is very strong. It takes a lot of hard work and a lot of time to use it to take part in the reaction to the life process, so there are many places on earth that are prone to phosphorus and "hungry" ecosystems.
the arsenic in the element periodic table below phosphorus is also mainly in the ore, but arsenic also has natural monomer, which is not more difficult to use than phosphorus, even in some places more abundant than phosphorus, such as Lake Monod. In fact, arsenic is toxic because it is too easy to be misused by organisms: arsenic, as a substance similar to phosphorus, often participates in biological metabolism, in the tube at normal temperature, AMP (adenosine monophosphate, two less phosphoric acid than ATP, one of the basic units of RNA) needs an enzymatic reaction to produce, and AMAs (single) Adenosine arsenate is easily generated automatically. Because arsenic compounds interfere with the process of life that is supposed to be involved in phosphorus, it is toxic, and the well-known poison, such as arsenic trioxide, is arsenic trioxide.
under normal conditions, why not accept arsenic as a component of vital substances mentioned earlier? Because arsenic compounds are not as stable as phosphorus compounds at normal temperature, if arsenic acid is used as a connector in the DNA molecule, the DNA chain is easily broken from arsenic acid. However, the GFAJ-1 strain, as a rare species of polar organism, makes arsenic safe to use: it may not replace all the phosphorus in the cell as arsenic, but they contain DNA of arsenic acid and even carry a series of processes extracted from the laboratory, and we don't know for a moment before the genome is analyzed in detail. How do they do it?
in fact, the discovery of arsenic in Monod lake is not news. As early as 2008, photochemical oxidation of arsenite acid instead of photolysis of water has been found. But the bacteria only use the arsenic compounds as the electrons in the light reaction to fix carbon dioxide, but the discovery of the GFAJ-1 strain is made up of a variety of key molecules of its own, which is called "arsenic based life", which has caused a sensation in the academic world.
the environment we fear may be thriving, uh, and vice versa...
scientists speculate that the life of arsenic is probably an ancient inhabitant on our planet. When phosphorus on the earth has not been transported to the world by the biosphere, perhaps many living organisms can eat only by arsenic, and the organisms that use arsenite to do photosynthesis may also appear earlier than the modern photosynthetic organisms. If the primitive earth is such a scene, what about the alien? We think the structure of the arsenic - based life is unstable, just at the temperature of our species and at the comfortable room temperature we feel, and what's the case at -180 degrees Celsius six?
those "unsuitable living" planets may be only because we know the limitations of our own wishful thinking, at least today, we know that the six major elements of life on earth that biologists have long been familiar with are likely to be replaced. "Science begins when you don't believe in experts." A guest at the NASA conference said.
finally, my sigh is that the periodic table is really a magic thing. I think of an old film called evolution: when extraterrestrial life evolves on earth and threatens human survival, the protagonist is inspired by arsenic toxicity, and in the periodic table the Head and Shoulders is found to deal with aliens, so a number of gallons of Head and Shoulders have successfully saved human beings. The weapon of the comedy, Zhongfei Fei, seems to be selenium. If there is one day, pure arsenic - based extraterrestrial life invades the earth, maybe a variety of phosphorus rich detergent can really become a drug that can poison the aliens. It is essential to travel home and kill people.